The Centers for Disease Control and Prevention issued a Health Alert Network advisory on July 23 about a critical Becton Dickenson blood culture vial shortage that will seriously disrupt patient care. They also held a clinician call with the Infectious Diseases Society of America, the American Society of Microbiology, the Society of Healthcare Epidemiology of America, and the Pediatric Infectious Diseases Society.
While this sounds esoteric—why should anyone care about these vials?—there is no question that this shortage will seriously hurt patients. Here’s why.
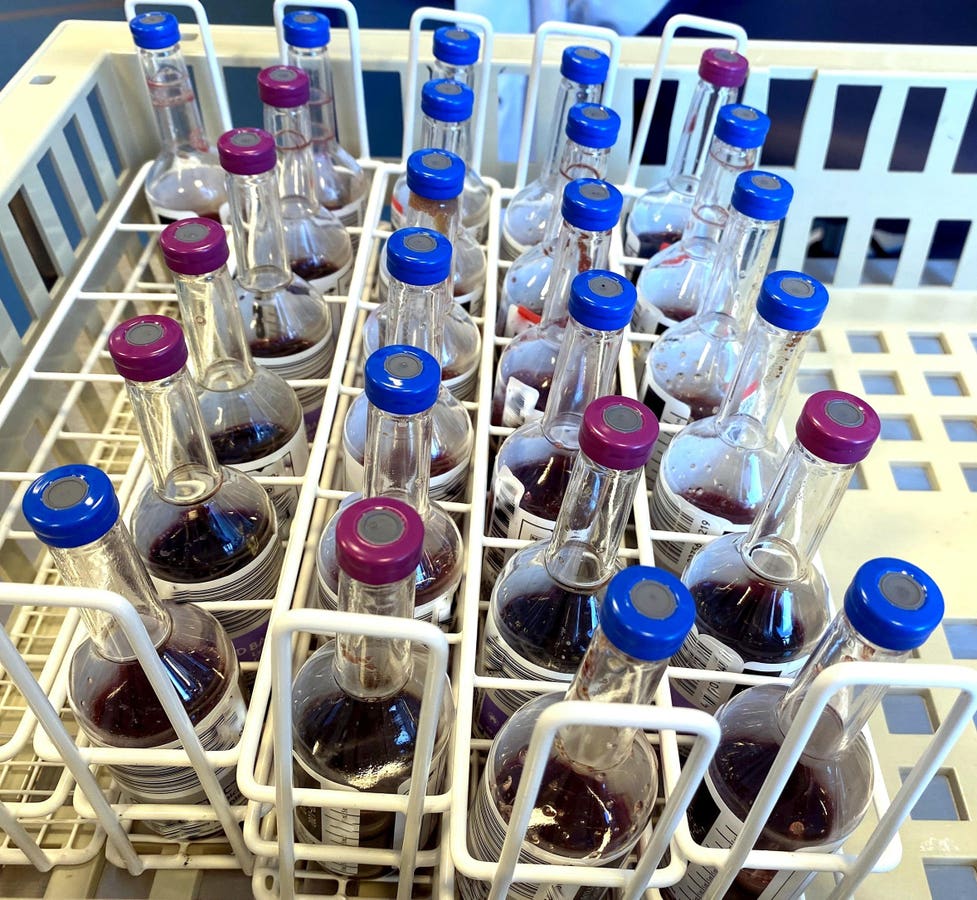
When a patient comes to the hospital with a serious infection or fever, one of the first things done is to draw two sets of blood cultures to try to identify the source of the infection and the antibiotics that will most specifically target the bacterial (or fungal) culprit. Because blood is normally sterile, most bacteria that grow in the blood culture are the source of the infection. Skin or stool bacteria can sometimes cause infection, particularly on heart valves or foreign bodies (like a prosthetic joint). It can occasionally be hard to tell if the bacteria is a contaminant from the culture site not being sterilized properly or is truly the pathogen. If this kind of infection is suspected, two or even three sets of cultures are drawn before antibiotics are started.
On this clincian call, Chris Beddard, vice president at BD Life Sciences, said the shortage could last into September. She alluded to a manufacturing problem with the source of the specially shaped plastic vials used for the culture bottles, but did not go into details.
Dr. Romney Humphries, medical director of Vanderbilt University Medical Center microbiology lab, startled some attendees by indicating how dire the situation was. They had only a few days notice of the shortage. She said that Vanderbilt “received a shipment of 10 individual bottles for the entire 1700-bed hospital.” Drawing only one set of cultures “is absolutely not the standard of care. But we’ve been forced into a situation where we can’t provide the standard of care for this shortage. So I think it does become a risk decision. Is no blood cultures for any patient worse or better than having one set for all patients?”
Several speakers on the call suggested reducing the number of cultures done by changing the criteria, such as not allowing repeat cultures in less than 48 hours. Others stressed meticulous attention to sterile technique to reduce the number of contaminants and the need for repeat cultures. Some proposed using expired bottles.
Note that cultures from one manufacturer are not interchangeable with those from others because of the shape of the bottle or the type of machine they works with. Bactec is a leader in the US market. In terms of revenue, the blood culture test market was estimated to be valued at $5.2 billion in 2023 and $7.6 billion by 2026. The top three companies in this niche are Becton Dickinson, Terumo, and bioMérieux.
Attendees raised several concerns, including standards of care. Currently, sepsis guidelines (and reviews for quality assurance) require that two sets of cultures be drawn, generally within an hour of arrival in the emergency room, and prior to the patient receiving antibiotics. Will physicians be penalized now for only drawing one set?
One participant said he hoped the government would publicly note that this is a situation where crisis standards of care and rationing will occur—both to inform the public and to reduce the likelihood of physicians being sued for malpractice for only drawing one set.
Marsha Steed is a microbiology expert who has also had the misfortune to have repeated sepsis with an antibiotic-resistant E. coli. After the call, which she, too, attended, she told me, “It was very, very scary,” and on a recent hospitalization, “they only pulled one bottle, and that kind of jumped out. I know usually, there are different bottles for different media that test for different types of bacteria. And they only use one bottle. And when the nurse was doing the draw, she didn’t say they were on shortage, but she had said, ‘I have to be really careful when I take the sample because I can’t go under the volume and I can’t go over the volume, they want you to be really careful.’” During the clinician call, they also mentioned possibly using anaerobic or Myco lytic (used for TB) bottles instead of the usual aerobic bottles. Steed responded, “You know, the aerobic ones are really what’s going to get probably a better result and a wider range of organisms.”
Matthew Wynia, MD, MPH, is an infectious disease specialist and bioethicist. We discussed the recurrent problem of relying on a sole source for a product. I’ve had long-standing concerns about a fragile supply chain and resultant shortages. A prime example is the shortage of the staple, IV saline, which occurred after the Baxter plant in Puerto Rico went off production because of hurricanes. They produced 43% of the US market’s IV saline needs.
Wynia noted, “there have been dozens of reports written about how to address drug shortages, all of which include the recommendation that we really ought not have sole source for anything. You need diversification of your supply chain.” He added that “just in time delivery of the cheapest possible product” contributes to the problem, as does this being “the equivalent of a generic drug.”
Wynia has long been concerned about moral distress caused by forced actions. He said, “We may never fully recover psychologically from the pandemic and the moral distress associated with having patients who needed something, and we couldn’t give it to them because we didn’t have it.” He sees the same thing happening now with this critical shortage of blood culture bottles. He also questioned what, if anything, patients will be told.
A final concern I raised is the use of unnecessarily broad-spectrum antibiotics to “cover” possible resistant organisms. This will predictably lead to superinfections with more resistant organisms, fungi, or C. difficile diarrhea. This was seen, for example, when a shortage of piperacillin-tazobactam led to an increase use of meropenem and subsequent C. diff.
But the bottom line is that this blood culture bottle shortage will result in difficult triage and rationing decisions, and both physicians and patients will be hurt, although in different ways.
Read the full article here